Patient-centred care:
turn the slogan into substance.
Health policies and programs are strongest when they are co-designed with the people they aim to benefit.
Real and constant engagement with patients at every step of the policy-making process should be the standard we strive for.
Challenge the status quo and use your voice as a driving force for change.
Whether you want to focus on access to treatment, quality of life initiatives or research funding, you can advocate for change on behalf of Australians affected by bowel cancer.
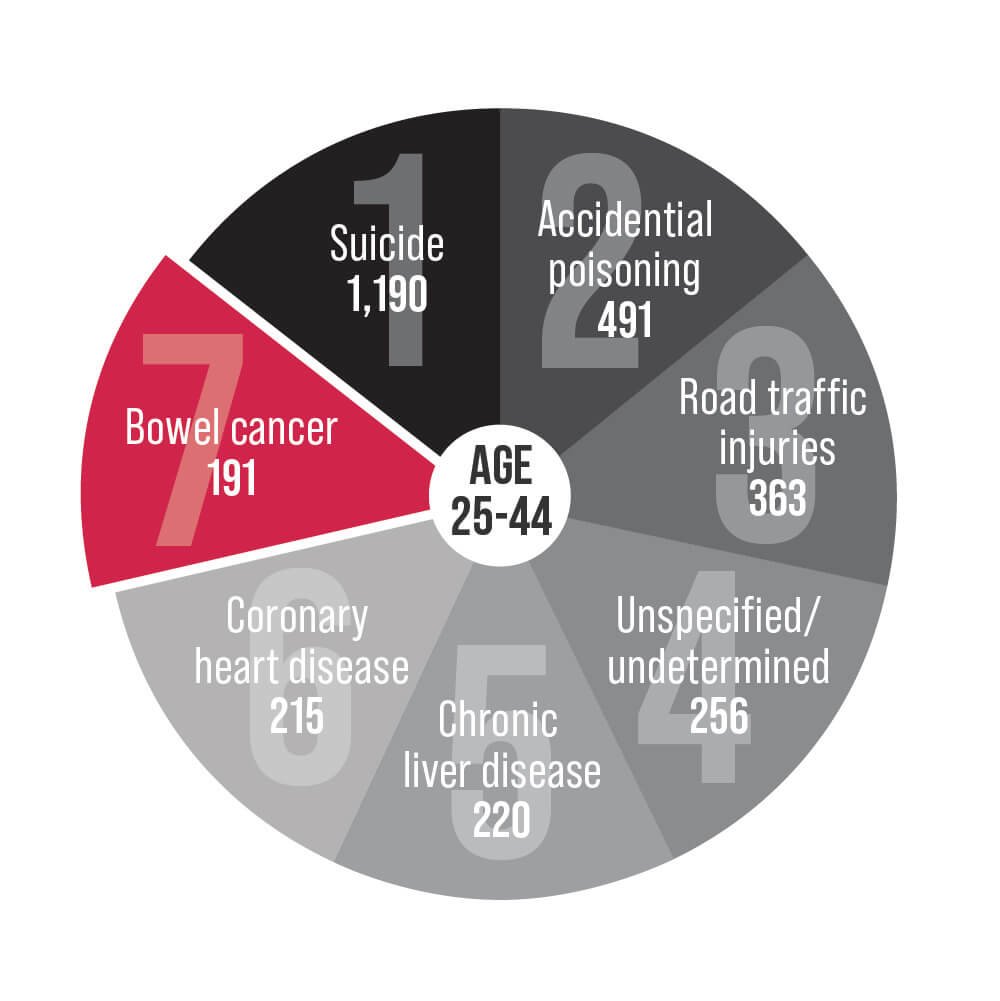
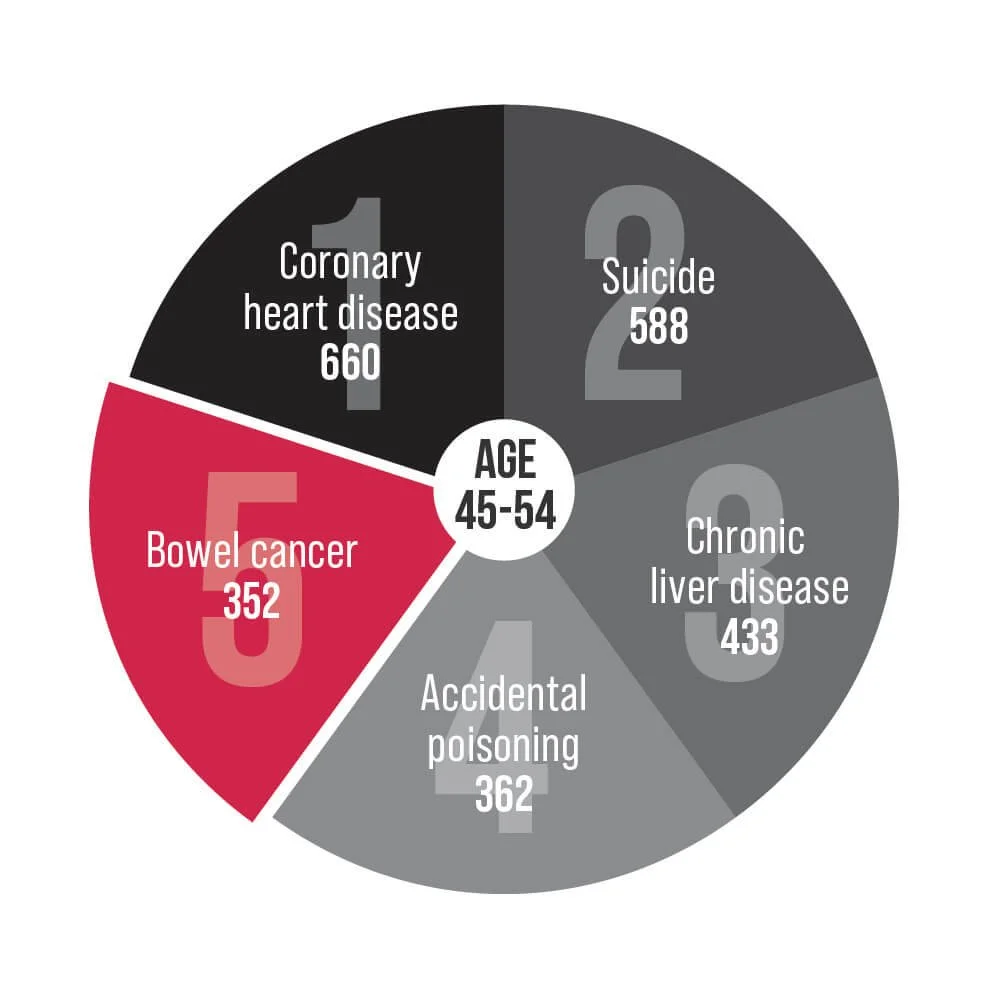
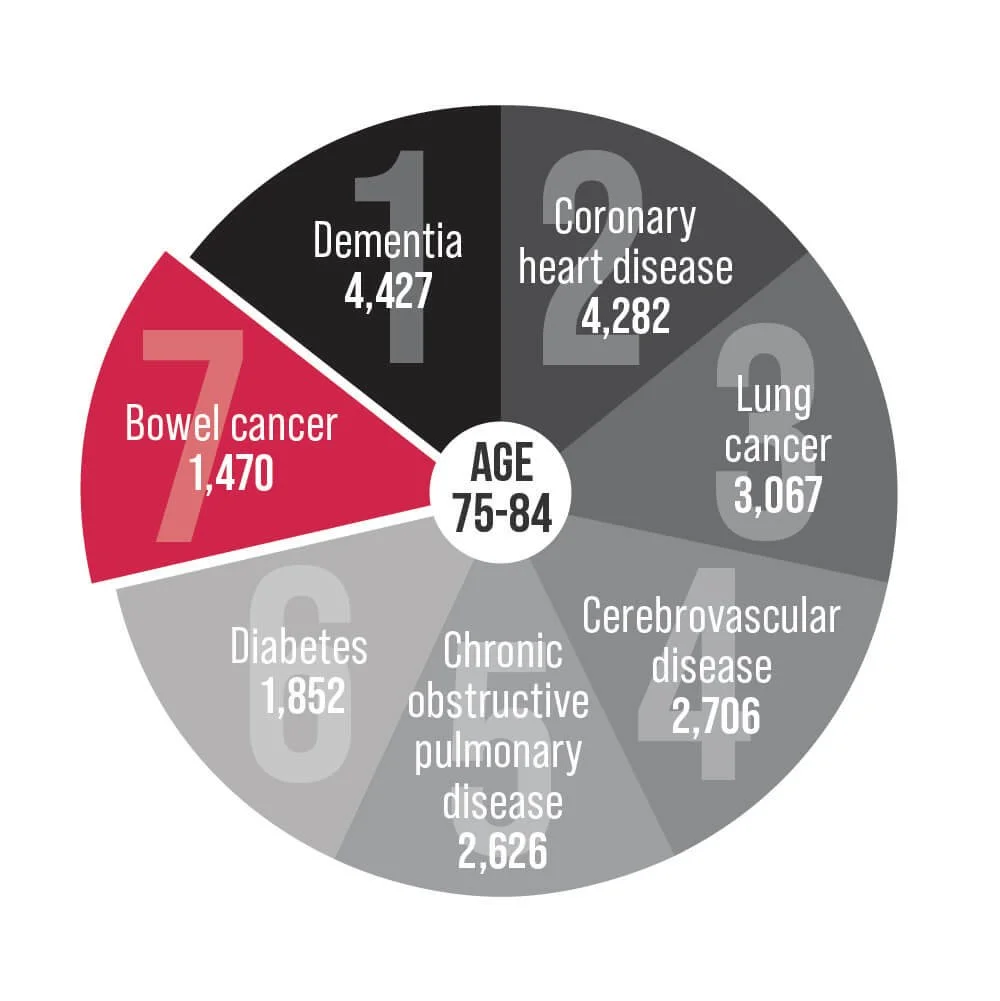
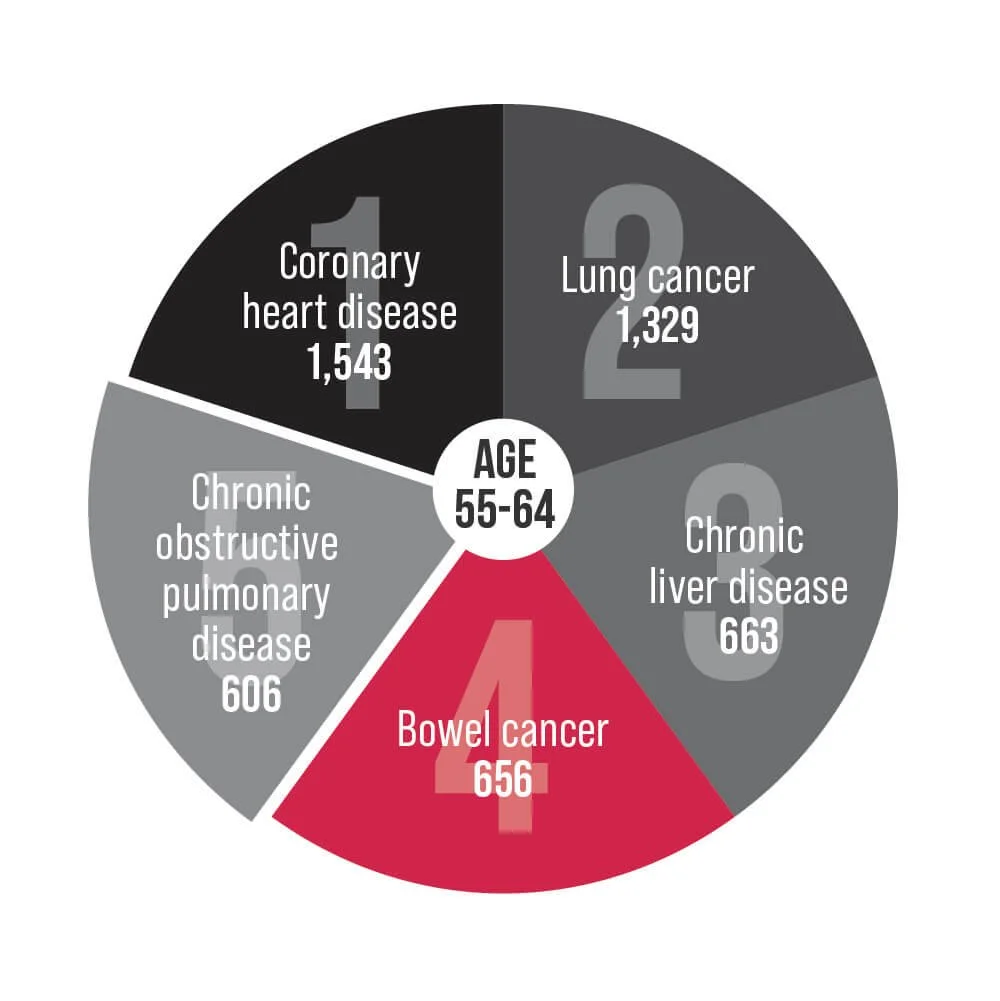
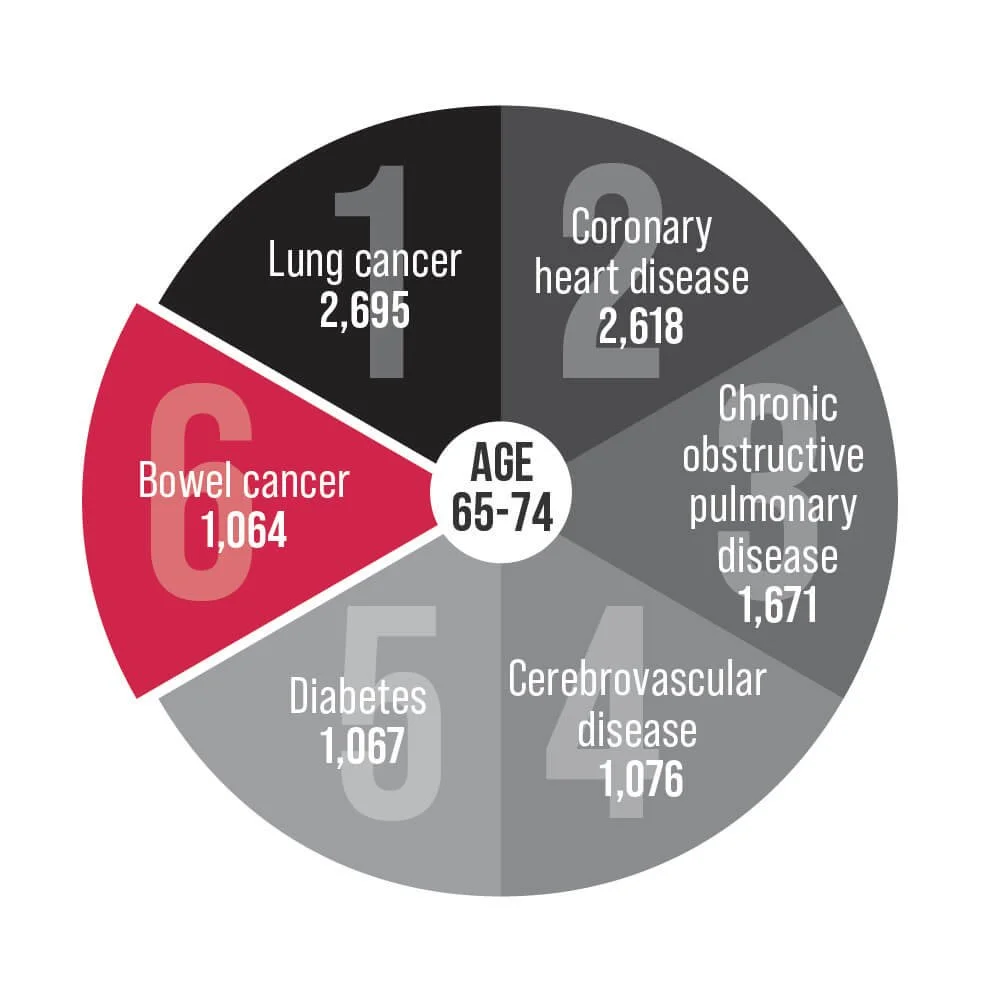
Bowel cancer is an underlying cause of death for Australians aged 25-84. Help us change that.
Colonoscopy wait-time guarantee
Wait times for those who received a National Bowel Cancer Screening Program (NBCSP) positive test result varied between 129 and 179 days depending on where participants lived.
Bowel Cancer Australia advocates for a colonoscopy wait-time and performance guarantee:
A colonoscopy within 30 days from first healthcare presentation for people experiencing symptoms suggestive of bowel cancer or a positive screen. If wait times exceed 120 days, a prognosis can worsen if cancer is present.
Transparency and public reporting of colonoscopy wait-times by all public and private healthcare facilities, released quarterly.
Adequate funding for colonoscopy services across Australia.
Collection of patient-reported experience measures within 30 days via a questionnaire from all people who undergo a colonoscopy, asking them about their pre-procedure experience (whether people understood the risks/benefits), the hospital experience (the procedure itself, issues of dignity/privacy); and post-procedure complications (bleeding/pain), with results publicly reported.
Minimum quality standards and key performance indicators (KPIs) for the delivery of colonoscopy within Australia, along with recording and public reporting of performance against the standards and KPIs.
Bowel Care Nurses
Results from Bowel Cancer Australia’s national patient survey, My Cancer, My Voice, revealed bowel cancer patients at all stages of the disease felt they had the 'wrong cancer', due to a lack of dedicated support services and low awareness of the disease.
Although bowel cancer is the fourth most diagnosed cancer and second deadliest, bowel cancer patients don’t receive the same level of support as other common cancers.
One-third of bowel cancer patients reported seeing between four and six health professionals and in 16% of cases, patients reported having to see more than seven health professionals when coordinating their care.
A Bowel Care Nurse is a registered nurse who has specialist knowledge and experience caring for patients with bowel cancer, and every bowel cancer patient deserves access to one.
Access to a nurse specialist by phone or in-person was identified by more than 8 in 10 (83%) patients as an important resource to improve their care coordination.
Since 2011 Bowel Cancer Australia has been providing specialist teleheath support services described as a 'lifeline' by patients and their loved ones, the service enables access to personalised care and tailored support nationwide.
In 2018, the charity has funded specialist in-person nurses in communities who act as a point of contact for bowel cancer patients and their families as they navigate the health system.
Bowel Cancer Australia’s in-person specialist Bowel Care Nurse locations
Early-onset bowel cancer
1 in 8 new bowel cancer cases now occur in Australians under age 50.
Bowel Cancer Australia’s Never2Young Advocacy Agenda seeks to improve care experiences and health outcomes for younger people by championing:
Greater awareness among the community and health professionals of early-onset bowel cancer.
Lower screening age below 50, in response to the increasing rates of bowel cancer in younger people.
Prompt GP referral for a colonoscopy for all people regardless of age, who present with symptoms that may be consistent with bowel cancer.
Improved pathways that ensure timely triage, diagnosis and treatment for younger people.
Better understanding the challenges faced by early-onset bowel cancer patients to improve and tailor treatment, support and care.
Further research into the causes of early-onset bowel cancer, to help build a path toward a cure.
Health Technology Assessment (HTA) Review
A process called HTA informs Government decisions on what medicines, medical services, and products to fund and subsidise.
The Australian Government has agreed to support an independent review of the current Health Technology Assessment (HTA) policy and the methods used by the Pharmaceutical Benefits Advisory Committee (PBAC) to assess new medicines for listing on the Pharmaceutical Benefits Scheme (PBS). The review will explore contemporary research and relevant methodologies and purchasing practices used by comparable international jurisdictions as part of the process.
The review will be overseen by a committee that is independently chaired, and includes a patient representative, a member nominated by Medicines Australia, a Government Nominee, the Chair of the PBAC and the independent Chair.
MBS item changes and colonoscopy services
The Government established the MBS Review Taskforce to address feedback received from clinicians and the broader community that some services on the MBS did not reflect clinical best practice.
The Taskforce’s colonoscopy recommendations became effective from November 2019, followed by chemotherapy recommendations in November 2020, with colorectal surgery recommendations scheduled for implementation in 2022.
The new Medicare item numbers for colonoscopy are the most dramatic change since they were created, and the impact of these changes was felt immediately.
While there are no restrictions for people accessing colonoscopy if they have new symptoms or a positive screening test result, many patients with a personal or family history of the disease have been affected, resulting in significant stress and anxiety.
Patients with between 1 and 4 sub-centimetre colonic tubular adenomas with low grade dysplasia will only be allowed one colonoscopy every 5 years.
Individuals with a family history, defined as a First Degree Relative (FDR) aged below 55, or 2 FDRs of any age, or one FDR and 2 Second Degree Relatives (SDR) of any age, are allowed one colonoscopy every 5 years.
From 1 November 2019, patients will be allowed only one colonoscopy per lifetime in special circumstances when a specialist is unable to access sufficient patient information and in the specialist’s opinion there is a clinical need for a colonoscopy.
The Medicare items and services are now being reviewed on an ongoing basis by a new Medicare Benefits Schedule (MBS) Review Advisory Committee (MRAC).
It is important the Committee understands your lived-experience and how these changes have impacted you.
The ‘Patient Access Gap’ – access to new treatments
The Patient Access Gap refers to the time in days patients must wait between the date a medicine is authorised for use in Australia (TGA approved/ARTG listed) and the date that it is listed on the PBS and affordably available.
Bowel cancer patients have experienced some of the longest waits, with one life-extending medication taking more than six years and a record eight submissions before being listed on the PBS as a subsidised treatment.
Cancer treatment is time sensitive, and many patients don’t have time to wait, which is why Bowel Cancer Australia advocates for immediate access following approval of a drug, so patients can benefit from treatments, particularly life-saving drugs, while PBAC processes and negotiations continue.
Making bowel cancer research a priority
Bowel cancer is Australia's second deadliest cancer and has the second highest disease burden of any cancer in Australia.
Burden of disease measures the impact of living with illness and injury or dying prematurely.
However, some cancers are funded disproportionately to their burden of disease.
Bowel Cancer Australia in collaboration with funding partners has committed $19.78 million to bowel cancer research projects, including a $10.4 million investment in the Lawrence Penn Chair of Bowel Cancer Research and mass spectrometry core facility, dedicated to leading edge bowel cancer discoveries; and $3 million to establish an Early-Onset Bowel Cancer Centre of Research Excellence - a focal point of expertise, to foster and facilitate national collaborative research endeavours focused on the cause (aetiology) of the increase in early-onset bowel cancer.